The Navajo Nation
Office of the President and Vice President
FOR IMMEDIATE RELEASE
December 9, 2020
191 new cases, 10,192 recoveries, and five more deaths related to COVID-19 as Pfizer vaccine seeks FDA approval on Thursday
WINDOW ROCK, Ariz. – On Wednesday, the Navajo Department of Health, in coordination with the Navajo Epidemiology Center and the Navajo Area Indian Health Service, reported 191 new COVID-19 positive cases for the Navajo Nation and five more deaths. The total number of deaths is now 693 as of Wednesday. Reports indicate that 10,192 individuals have recovered from COVID-19, and 176,602 COVID-19 tests have been administered. The total number of positive COVID-19 cases is now 18,575, including 60 delayed reported cases.
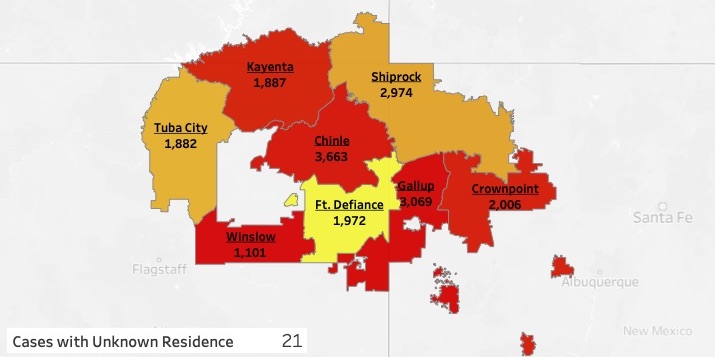
Navajo Nation COVID-19 positive cases by Service Unit:
- Chinle Service Unit: 3,663
- Crownpoint Service Unit: 2,006
- Ft. Defiance Service Unit: 1,972
- Gallup Service Unit: 3,069
- Kayenta Service Unit: 1,887
- Shiprock Service Unit: 2,974
- Tuba City Service Unit: 1,882
- Winslow Service Unit: 1,101
* 21 residences with COVID-19 positive cases are not specific enough to place them accurately in a Service Unit.
On Wednesday, the state of Utah reported 2,574 cases, Arizona reported 4,444 new cases, and New Mexico reported 1,759.
Navajo Nation President Jonathan Nez and Navajo Department of Health Executive Director Dr. Jill Jim spoke with Pfizer CEO Dr. Albert Bourla on Wednesday, regarding the pending approval of Pfizer’s vaccine for COVID-19. The U.S. Food and Drug Administration is expected to vote on approving the Pfizer vaccine on Thursday, which will then allow for the distribution of the Pfizer vaccine based on the CDC’s plan to first prioritize health care workers and those living in long term living facilities with initial distributions. Pfizer has reported that its vaccine is 95-percent effective against COVID-19 with no serious safety concerns.
Earlier this year, the Navajo Nation Human Research Review Board, the National Indian Health Service IRB, and the Johns Hopkins School of Public Health IRB approved the Pfizer COVID-19 vaccine clinical trial. As of Dec. 8, over 43,000 people have volunteered for the Pfizer vaccine trials worldwide, including members of the Navajo Nation and the White Mountain Apache Tribe. Moderna is also seeking approval from the FDA for its COVID-19 vaccine, which has also shown 94.5-percent effectiveness. Navajo Area IHS is developing plans for the distribution of the vaccines on the Navajo Nation, if or when the vaccines are approved by the FDA.
“In order to drastically reduce the risks and spread of COVID-19, we need a safe vaccine. We have lost far too many of our people and the spread of the virus has significantly increased recently. Approximately 150 residents of the Navajo Nation volunteered for the vaccine trials and I have not received any reports of any major side effects or concerns. Until a safe vaccine is widely available, we have to continue to fight this virus together and the best way to do that is by staying home as much as possible. The safest place during this pandemic is at home here on the Navajo Nation, stay local, stay safe,” said Navajo Nation President Jonathan Nez.
The Nez-Lizer Administration will host an online town hall on Thursday, Dec. 10 at 6:00 p.m. (MST) on the Nez-Lizer Facebook page to provide more updates.
“There is a light at the end of this dark tunnel, but until we reach that light, we have to remain strong and continue to help one another get through these challenging times. Please keep praying for those who have the virus, those who have lost loved ones, and for our first responders who are fighting for us every day,” said Vice President Myron Lizer.
For more information, including helpful prevention tips, and resources to help stop the spread of COVID-19, visit the Navajo Department of Health’s COVID-19 website: http://www.ndoh.navajo-nsn.gov/COVID-19. For COVID-19 related questions and information, call (928) 871-7014.
Indian Health Service Announces New Deputy Director for Quality Health Care and Enterprise Risk Management (Indian Health Service)
Federal Emergency Management Agency (FEMA)
White House Office of Management and Budget (Joe Biden Administration)
Tuba City Regional Health Care Corporation (Arizona, Navajo Nation)
Oklahoma City Indian Clinic (OKCIC)
Indian Health Service (Department of Health and Human Services)
Navajo Nation Town Hall (Arizona, New Mexico, Utah)
Navajo Nation (Arizona, New Mexico, Utah)
Tribal organizations statement on advance appropriations for Indian Health Service
Indian Health Service Statement on Advance Appropriations (Department of Health and Human Services)
Indian Health Service (Department of Health and Human Services)
Indian Health Service (Department of Health and Human Services)
Navajo Nation (Arizona, New Mexico, Utah)
Indian Health Service (Department of Health and Human Services)
