FOR IMMEDIATE RELEASE
Grayson Corporation Now Offers COVID-19 Rapid Antibody Test with Emergency Use Authorization by U.S. Food and Drug Administration (FDA)
Rapid Antibody Testing may be used to identify whether people were previously infected by COVID-19, including those that may be asymptomatic or have recovered.
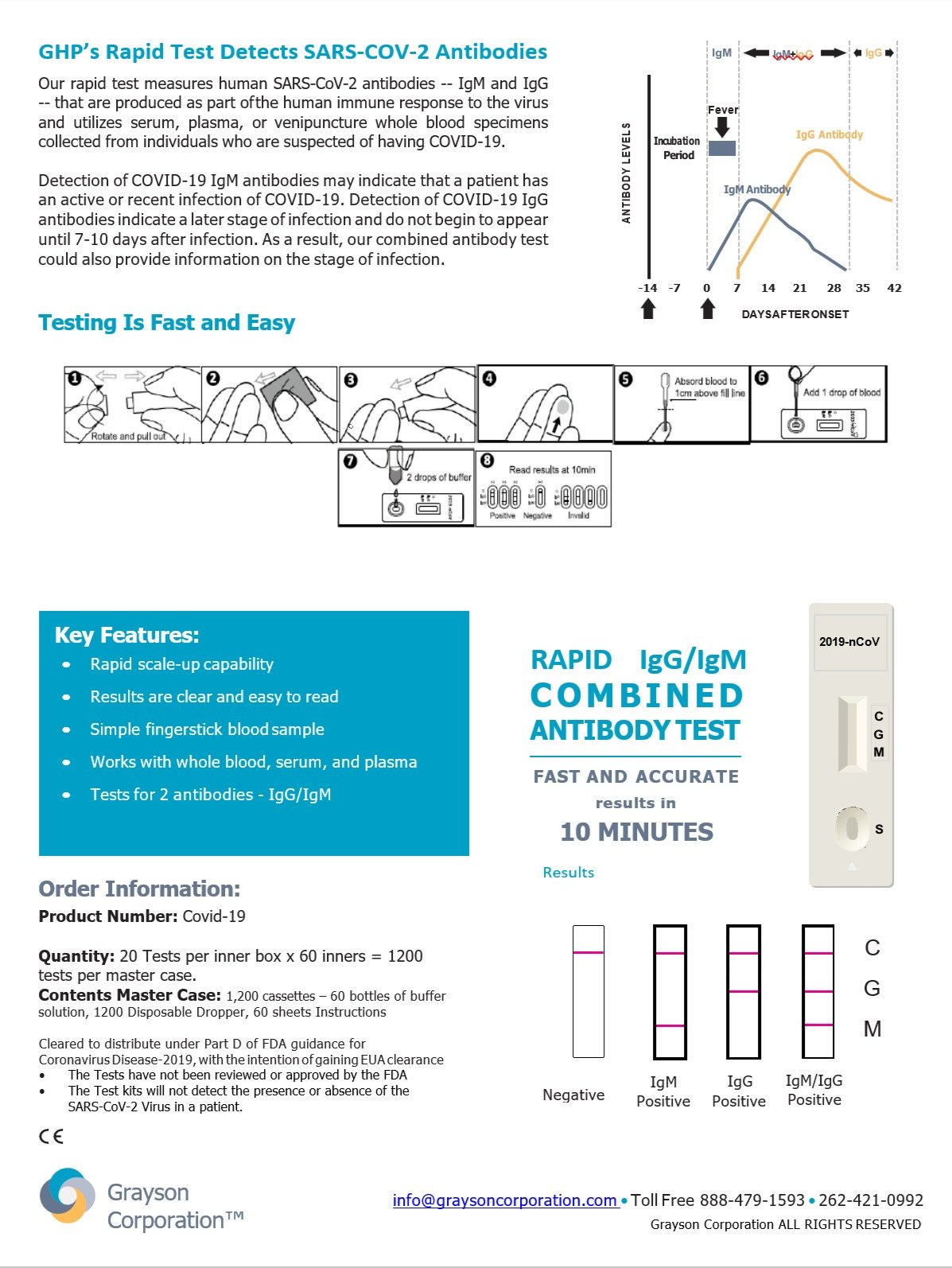
LIBERTYVILLE, Ill., June 29,2020 – Grayson Corporation announced that they now offer a Rapid Antibody Test for COVID-19 diagnostic testing with Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA). The COVID-19 Rapid Antibody Test allows testing of a sample collected from blood, serum, or plasma using the kit that contains a cassette, buffer, dropper, and instructions to have results in 5 to 15 minutes. Grayson Corporation’s COVID-19 Rapid Antibody Test is a simple finger-stick cassette that tests for two antibodies, IgG/IgM, which can be used anywhere with no specialized training or equipment required.
Detection of COVID-19 IgG/IgM antibodies may indicate that a person has an active or recent infection of COVID-19. These antibodies indicate a later stage of infection and do not appear until 6-10 days after infection. The IgG antibody is a protein that the body produces in the late stages of infection. This antibody may remain in the body for several months or possibly years after recovery.
With Grayson Corporation’s ample manufacturing capacity, the company will be able to distribute several million units in the coming weeks. “Rapid Antibody Testing is an important tool for the assessment of immunity to COVID-19. These tests will allow healthcare professionals and employers to provide on-site wellness checks for patients and employees as we begin the new phases of re-opening the country. Our Rapid Antibody Tests will help with community mitigation strategies and protect others from potential exposure.” said Don Ryan, Chief Executive Officer of Grayson Corporation.
Grayson Corporation is a Medical Supply distribution company based in Libertyville,Ill. that specializes in delivering high quality medical products to professionals and consumers. Founded in 2014, Grayson Corporation looks to develop innovation in healthcare products.
For more information or for purchasing the test please contact Mary Bradley at 616-822-9118 or email: mary@graysoncorporation.com.
Tuba City Regional Health Care Corporation (Navajo Nation)
HHS Small Ambulatory Program Awards $55 Million to 15 Tribes and Tribal Organizations (Indian Health Service)
Indian Health Service Announces New Deputy Director for Quality Health Care and Enterprise Risk Management (Indian Health Service)
Federal Emergency Management Agency (FEMA)
White House Office of Management and Budget (Joe Biden Administration)
Tuba City Regional Health Care Corporation (Arizona, Navajo Nation)
Oklahoma City Indian Clinic (OKCIC)
Indian Health Service (Department of Health and Human Services)
Navajo Nation Town Hall (Arizona, New Mexico, Utah)
Navajo Nation (Arizona, New Mexico, Utah)
Tribal organizations statement on advance appropriations for Indian Health Service
Indian Health Service Statement on Advance Appropriations (Department of Health and Human Services)
Indian Health Service (Department of Health and Human Services)
Indian Health Service (Department of Health and Human Services)
